X-ray Absorption Spectroscopy ( XAS)
The sudden rise in absorption at the edge occurs when an incident x-ray photon has just sufficient energy to cause transition of an electron from the
1s state of some element to an unfilled state of predominantly p-character at the K-edge (i.e. angular momentum l = 1 with respect to the central absorbing atom).
In the near edge region, sometimes called the "X-ray Absorption Near Edge Structure'' (XANES), transitions may occur to unfilled bound states,
nearly-bound states (resonance), or continuum states of the appropriate symmetry. Well above the absorption edge ~30 eV, in the
"Extended X-ray Absorption Fine Structure'' (EXAFS) region, transitions are to continuum states. Edges due to transitions from less deeply bound levels
(e.g. 2S, 2P1/2 , 2P3/2 , 3S..., which are designated LI ,LII , LIII , MI ...
edges) also occur at lower x-ray energies (soft x-ray).
It has become clear in recent years that there is no fundamental distinction between the physics of EXAFS and XANES; the distinction is only one of complexity
of the spectra. For example, effects such as the energy dependence of the central atom absorption and multiple scattering are more important in the XANES
region. Modern theories appear capable of explaining the entire spectral range within the context of multiple scattering theory. For this reason EXAFS and XANES
are now referred to jointly under the term "XAFS''.
It should be noted however that the structure in the pre-edge region and on the rising part of the edge often
is rather sensitive to details of the local site symmetry, bond length, charge state, and orbital occupancy. This XANES information can often be exploited to
provide information on the chemical state of the sample, even in case where EXAFS spectra cannot be obtained with adequate signal to noise ratio.
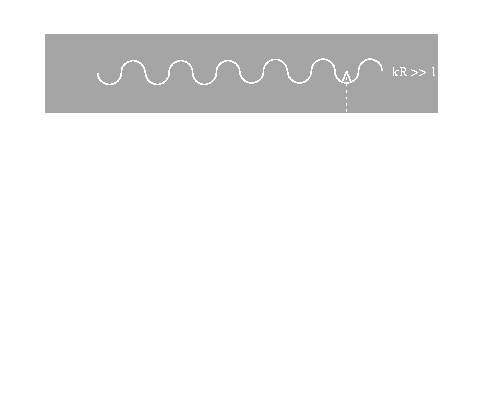
On the other hand, EXAFS refers to the sinusoidal variation of the x-ray absorption coefficient as a function of x-ray photon energy, which occurs after each
absorption edge of an element and extends for up to 1200 eV further. This phenomenon forms the basis of the analytical technique of EXAFS spectroscopy,
which can be used to obtain structural information such as coordination number, interatomic distance and disorder about materials without the need for obtaining
single crystals.
Over the last two decades XAFS spectroscopy has emerged as an incisive probe of the local structure around selected atomic species in solids,
liquids, and molecular gases. Foremost among its strengths are its applicability to amorphous or layered materials and its "tunability'', that is, the ability to probe
the environments of different elements in the sample by selecting the incident x-ray energy.
Simple models of x-ray absorption predict a gradual monotonic decrease in the absorption coefficient with increasing energy away from
the absorption edge. Such behavior is observed in spectra of isolated atoms, e.g. noble gases (Xe, Kr etc.), but for atoms either in a molecule or a condensed
phase, the presence of other atoms around the absorber causes oscillations in the absorption coefficient near the edge. These oscillations in the post edge
region arise from the back-scattering of the emitted electron wave off/on neighboring atoms and so the structure of the post edge region of the x-ray absorption
spectrum is related to the radial distribution of atoms in the sample. Hence, by analyzing this fine structure, information about the local environment of the
absorbing element can be derived.
The amount of information available from a single XAFS spectrum (typically 10-20 or so parameters) is small compared
to that available from x-ray diffraction, but the information available from a well-chosen experiment can be particularly incisive and may be inaccessible by
any other technique. The main deficiencies of the technique are the sensitivity only to short range order, generally to ca. 0.4nm away from the
absorbing element, the lack of fully three dimensional information, and the large amount of data reduction and analysis involved. However XAFS
is particularly powerful when intimately combined with complementary techniques such as x-ray diffraction as a long range order technique or other
spectroscopic methods. Recent developments in theory and experiment show great promise for extending the range and power of the XAFS.


